by Guy S Eakin
While research into the causes of Lipedema progresses, we know patients and health care workers are still looking for what can be done today to improve the lives of people with Lipedema. This is often a trial and error process. Unfortunately, the trials and errors are not just in trying the right treatment, but even in finding which treatment to try. The level of poor information out there for women with Lipedema is tremendous, and often a therapeutic approach to Lipedema includes sifting through mountains of testimonials and anecdotes to find reliable data. As we bring 2020 to a close, we are taking a look at the Lipedema research that particularly excited us this year because of what it’s doing to help control that problem.
We would like to start our last blog for the year by pausing to celebrate a new study that carefully evaluates treatments Lipedema patients are already receiving. When we review research, we often look for specific things. In this article, we were looking for words and phrases like “statistical power,” “blinding,” and “effect size.” These loosely translate to: “Was the study large enough?”, “Did the investigators reduce opportunities for bias?”, and “Was the effect of the treatment large enough that a patient might notice the difference?”
This new paper by Tuğba Atan [1] was able to answer those questions by studying women who participated in six weeks of exercise and Complete Decongestive Therapy (CDT). While a growing body of research has suggested the benefits of CDT, this may be the first study where patients were randomized to different interventions, and the investigators did not know which intervention each patient received. The results were straight-forward. In advanced stage Lipedema, a combination of exercise and CDT were effective in reducing the size of women’s limbs, reducing pain, and improving their physical function. We don’t know whether this will also be true of women with lesser staged Lipedema, and we don’t learn if the effects are maintained. But we do hope that the study offers hope and new confidence to patients and physicians sifting through the research.
Two more papers that excited us this year cover stem cells, a household topic that captured public imagination more than 20 years ago when human embryonic stem cells were first reported. Over the subsequent years, the world seized on an almost miraculous promise to regenerate lost tissue, as well as the ethical considerations that followed. The work we’re talking about today often mentions stem cells, but it’s important to note that these cells are derived from adult, not embryonic, tissues. In fact, when we think about the term “stem cell,” there is a lot of room for confusion. For instance, there is a movement to begin talking about these cells as “medicinal signaling cells (MSC)” rather than “stem cells” [2]. While this abbreviation preserves that of the stem cell’s (SC) it focuses attention away from the idea that they might be used to regenerate healthy tissue, and instead onto the more practical idea that their value may be in the molecular signals they release.
Before we even get to a medicinal signaling cell, let’s talk about one specific class of MSC, adipose stem cells (ASCs). These ASCs can be found in very small quantities inside adipose tissue, or fat, and can typically be isolated from lipoaspirate, the by-product of liposuction, which is a somewhat common therapy for Lipedema. Adipose cells are one of several cell-types involved in Lipedema, and they are believed to behave differently than otherwise healthy fat. In Lipedema patients, we expect to see fat exhibit at least three qualities, especially in the legs: (1) its cells grow in size, (2) its cells multiply, and (3) the stem cells themselves generate new adipose tissue.
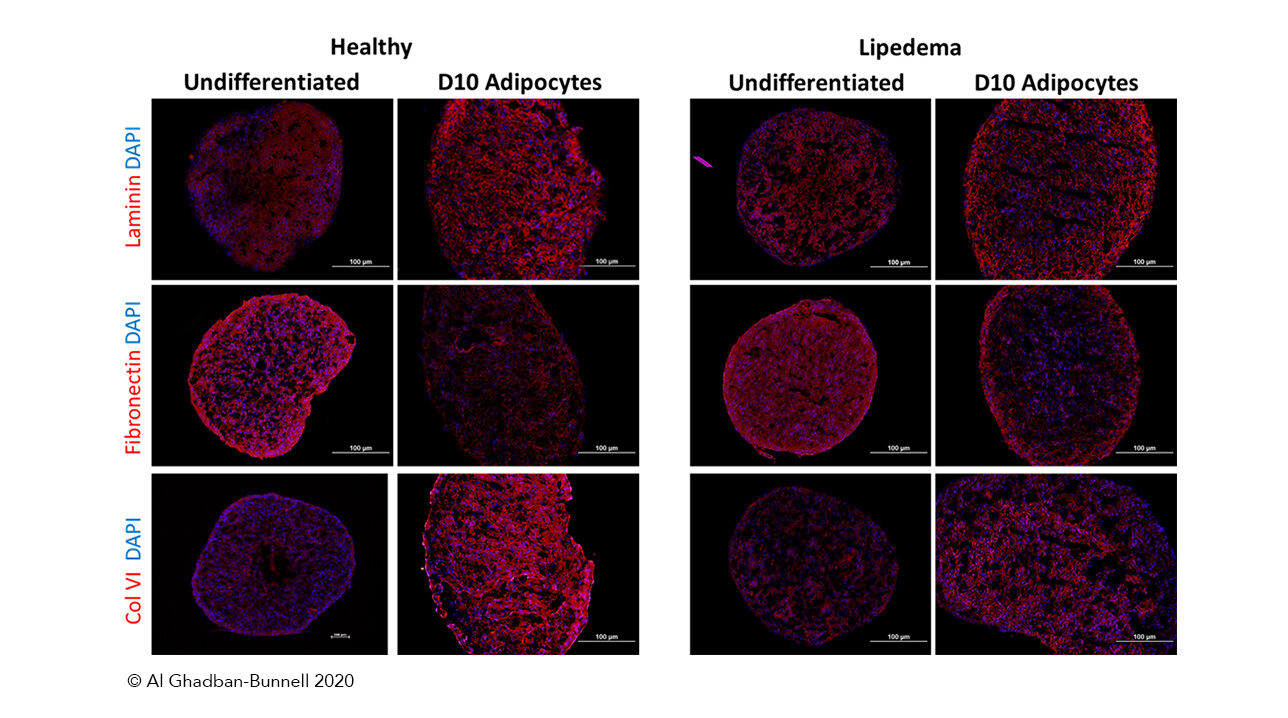
Lipedema is complex, so what happens when we remove adipose tissue from its environment? Will they still exhibit any of these three qualities? This year, Sara Al-Ghadban and colleagues examined this question by studying lipoaspirate, including tissue from women that are part of the Lipedema Foundation’s 25cohort [3, 4]. This is a generous and dedicated group of patients who are helping the field of Lipedema research by allowing their tissues and clinical data to be used and compared across many studies.
From this lipoaspirate, Dr. Al-Ghadban and her colleagues derived ASCs and then set their experiments up in a way that allowed them to compare ASCs not only between Lipedema and healthy controls, but also between adipose tissue in two different areas - the abdomen and the thigh - of the same patient.
Their results do, in fact, replicate the three qualities of growth, multiplication, and renewal. Initially they were able to see evidence that Lipedema thigh-derived ASCs grew more quickly and tended to differentiate more readily than abdominal-derived ASC. These differences were not seen when healthy control thigh and abdominal ASCs were compared.
They followed this work by taking the ASCs that had been growing on two-dimensional petri dishes and coaxing them to form small spheres. This 3D quality more closely resembles the environment in which the cells would live in an actual human body. In this experiment, the cells continued to grow and maintain their stem cell-like qualities.
By publishing this research, Dr. Al-Ghadban may have created an experimental model for Lipedema. This experimental ‘leg-in-a-dish’ model better mimics the behavior of cells in the body than others we’ve seen. While these cells will never be injected back into a human, they show us the promise of what stem cell technologies might bring to women in Lipedema. We can now explore questions about how fat tissue behaves in Lipedema quickly and can do so in a way that allows us to prioritize studies that require human volunteers. In the future, cells like these may also be a tremendous resource in testing how Lipedema fat responds to new medicines.
This final paper of our 2020 recap illustrates our excitement about the progress of Lipedema research: We leave the old year with new ideas and new tools that hold terrific promise for what we can achieve in the new year and beyond.
Looking forward to 2021, the Lipedema Foundation is committed to its New Year’s resolutions. As the largest funder of Lipedema research, we resolve to continue helping you make sense of the research landscape as it continues to grow. We resolve to push our field forward. And we resolve to start right away by launching a call for new research proposals in the first days of the new year.
We hope you’ll resolve to be part of a Lipedema movement as well, by doing just a few simple things throughout the coming year. Stay connected with us here and through social media. Sign up for alerts as new research becomes available. And consider completing a profile in the Lipedema Foundation Registry, and inviting a friend to complete one, too.
From all of us at the Lipedema Foundation, we wish you joy and happiness this season, and a wonderful start to a new year.